Expanded Recall for Baby Powder Issued

Dynarex Corporation is expanding the recall initiated on September 19, 2024External Link Disclaimer, to include an additional 373 cases of item number 4875, Dynacare Baby Powder, 14 oz., as well as 647 cases of item number 4874, Dynacare Baby Powder, 4 oz., because they have the potential to be contaminated with asbestos. Asbestos is a naturally occurring mineral that is often found near talc, an ingredient in many cosmetic products. Asbestos, however, is a known carcinogen and its health risks are well-documented.
Ohio is included in the list of states the affected product was shipped to on or after January 18, 2024. The product was also sold online on Amazon.
There have been no illnesses or adverse events reported to date.
The recall was the result of a routine sampling program by the FDA, which revealed that the finished products contained asbestos. Upon further investigation, the FDA has identified additional lots of products that may contain asbestos due to using the same bulk talc material. The company has ceased the distribution of the product as an investigation is proceeding to determine what caused the contamination of the talc.
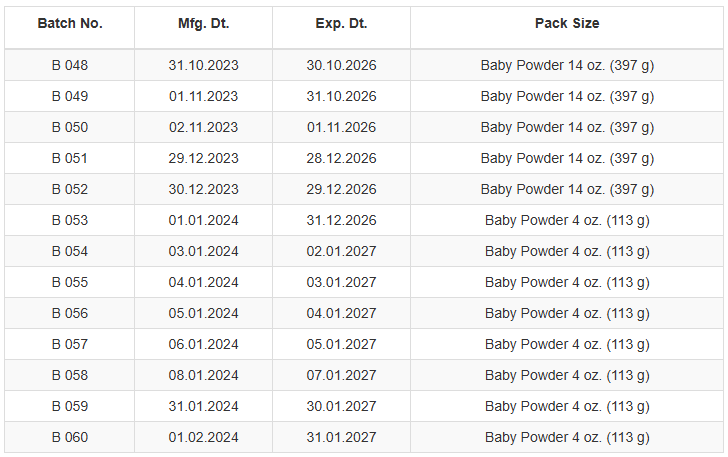
Consumers who have purchased Dynacare Baby Powder (see products/lots below) should discontinue use immediately and return it for a full refund.
Please contact Dynarex Corporation at 888-396-2739 or 845-365-8200 during business hours of 8:30 AM to 5:00 PM Eastern Standard Time, or via email at [email protected] if you have any questions or need information on how to return the product or receive a full refund.






